Health food belongs to special food, which requires registration or filing before coming into the market. In recent years, with the improvement of people's lives and the enhancement of health awareness, categories of health food are becoming more and more diversified, with new product emerging in endlessly. Antion summarized the registration and filing of health food in 2021 to help enterprises better understand the status of the health food industry.
Note: The data in this article comes from the registration and filing information announced by the SAMR and administration for market regulation in various provinces, autonomous regions and municipalities from January 1, 2021 to December 31, 2021.
1. Number of Health Food under the Registration System
In 2021, the CFE announced 2,339 health foods under the registration system, involving new registration, extension of registration, change of registration, technology transfer, etc. Among them, there are 11 imported health foods, mostly from the US and Japan, and 2,328 domestic health foods, mostly from Guangdong and Shandong.
2. Number of Health Food under the Filling System
As of December 31, 2021, the CFE and administration for market regulation in various provinces, autonomous regions and municipalities announced 2,401 health foods under the filing system. Among them, there are 2,359 domestic health foods and 42 imported health foods.
In terms of the country or region where the applicant of imported health food is located, the country with the largest number is the US, with 14 products, followed by Canada and Japan with 13 and 9 products respectively.
USA Bornatop Investment & Management Co., Ltd. has the largest number of imported health food under the filing system with 11 products, Jamieson Laboratories Ltd. from Canada ranking second with 7 products, and Nutricorp International from Canada and I.B Co., Ltd. from Japan sharing the third place with 6 products for each.
3. Dosage Form of Health Food under the Filing System
Permitted dosage forms for filing of heath food include tablet, capsule, oral liquid, granule, gel candy and powder. Tablets include swallow tablet, chewable tablet, lozenge and effervescent tablet. Capsules include soft capsule and hard capsule. In terms of the dosage form of filed products, tablet ranks first with 1259 products, accounting for 52.4% of the total, among which swallow tablets are the most. It is followed by capsule with 752 products, accounting for 31.3% of the total, most of which are soft capsules.
In January 2021, with the release of Dosage Form and Technical Requirements for Filing of Health Food (2021 edition), 2 new dosage forms of health food, powder and gelatinized candy, were approved for use. In the current list of health food under the filing system, powder can be used for vitamin and mineral supplement products and cell-wall broken ganoderma lucidum spore powder, and gelatinized candy can be used for vitamin and mineral supplement products. The number of products in the form of powder and gelatinized candy is 40 and 22 respectively for the year 2021. The filing of products in different dosage form is as follows:
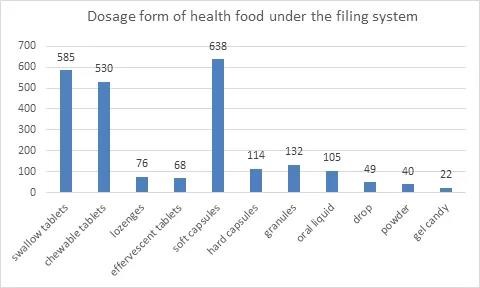
4. Function of Health Food under the Filing System
Health food under the filing system includes vitamin and mineral supplement products and products with specific health function. Among vitamin and mineral supplement products, single-nutrient supplement is the most with 1180 products, accounting for 49.1% of the total, while two-nutrient supplement is the least. Vitamin C supplement makes up the largest part of single-nutrient supplements with 388 products.
Ingredients of functional health food under the filing system include coenzyme Q10, cell-wall broken ganoderma lucidum spore powder, spirulina, melatonin and fish oil. Since the approval of the above 5 ingredients on March 1, 2021, 276 related products have been filed nationwide, among which cell-wall broken ganoderma lucidum spore powder is the most used. The filing of products with the 5 ingredients is as follows:
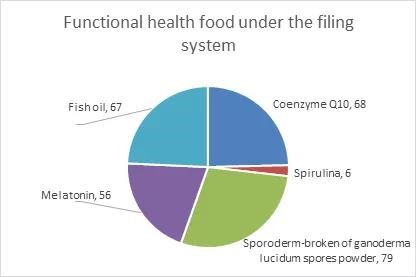
Recently, the SAMR released several drafts for comments including the health function list of nutrient supplement and non-nutrient supplement, ingredient list of nutrient supplement, and functional test and evaluation method of health food. With the release and implementation of formal regulations, health food industry will become more and more standardized, and there will also be new changes in the market pattern. Antion Consulting will pay continuous attention to the registration and filing of health food, to help enterprises better grasp the trend of the industry. In the meantime, we also provide standards and regulations consultation, food label audit, food related registration and application and other services. Please feel free to contact us if you have any questions.
Hongtao Fei
Tel: 010-51301566
email: feiht@ieasytrip.com
Source: Antion
Note: This article is compiled by Antion, please indicate our source if reprint it.