Health foods are special foods and need to be registered or filed before they can be marketed. If the raw materials of health foods have been listed in the catalogue of health food raw materials, the health foods are supplemented with vitamins, minerals and other nutrients and are imported for the first time, they need to be filed. The filed product formula, name and use amount of raw and auxiliary materials, efficacy and production technology shall comply with laws, regulations, rules, mandatory standards and technical requirements of catalogue of health food raw materials. Antion summarized the health food filing status of China in the first half of 2021 to help companies better understand the current health food filing status.
As of June 30, 2021 (the time of announcement), the official websites of CFE of SAMR and the market supervision administrations of provinces, cities, and autonomous regions have published a total of 417 filing health foods, which are all domestically produced.
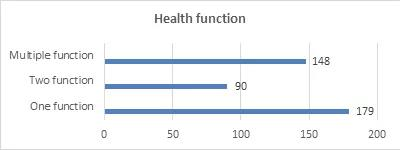
Filed Health Functions
In terms of the filed health functions, the single-function products have the largest number of filings, with a total of 179 kinds. Among them, there are 128 kinds of vitamin supplement products, accounting for 71.5% of the total. Among the vitamin supplement products, vitamin C supplement products have the largest number of filings, with a total of 88 kinds, accounting for 68.75%.
Among the single-function products, there are 46 products minerals supplement products, accounting for 25.84% of the total. The supplemented minerals include calcium, selenium, zinc and iron, and calcium supplement products account for the highest proportion.
Most of the products filed with two or more functions (that is, multiple functions) are mixed with vitamins and minerals. The filing of health functions is as follows:
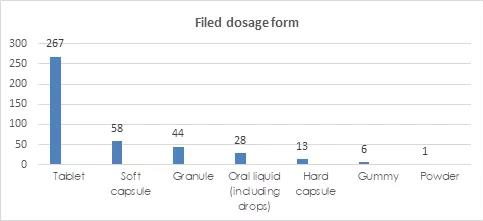
Filed Dosage Forms
The permitted dosage forms of filing health food include tablets, capsules, oral liquids, granules, gummy and powders. In terms of the dosage forms of filing products, tablets have the largest number of filings, with a total of 268 kinds, accounting for 64.27% of the total. Tablets include oral tablets, chewable tablets, effervescent tablets, lozenges and other types, with oral tablets being the majority.
Capsules include soft capsules and hard capsules. In the first half of the year, there were more soft capsules than hard capsules, 58 kinds and 13 kinds, respectively.
The Dosage Forms and Technical Requirements for Filing Health Foods (2021 Version) issued on January 29, 2021 supplemented powder and gummy. Products that use vitamins and minerals included in the catalogue of health food raw materials as raw materials can use these two dosage forms. In addition, the broken Ganoderma lucidum spore powder can also be used as powder. In the first half of 2021, a total of 6 kinds of gummies and 1 powder have been filed nationwide. The powder is broken Ganoderma lucidum spore powder.
Note: The filing data summarized in the article comes from filing certificates released in 2021, and does not include the filing certificates for changes and continuations. All filing numbers start with “Filing of health food 2021”.