On November 24, 2020, the State Administration for Market Regulation issued a notice for public comments on the Catalogue of Health Functions Allowed for Health Food Claims Non-Nutrient Supplements (2020 Edition) (Draft for Comments), which includes Evaluation Method of Health Food Functions (2020 Edition) (Draft for Comments), Interpretation of Health Food Function Claims (2020 Edition) (Draft for Comments) and Guidelines for Evaluation of Health Food Functions (2020 Edition) (Draft for Comments), and Guidelines for Ethical Review of People Trial of Health Food (2020 Edition) (Draft for Comments). The deadline for comments is December 23, 2020. In order to help enterprises better understand the relevant requirements, Antion will compares the Draft with the current version, and analyze and interpret the main changes and impacts of the Draft.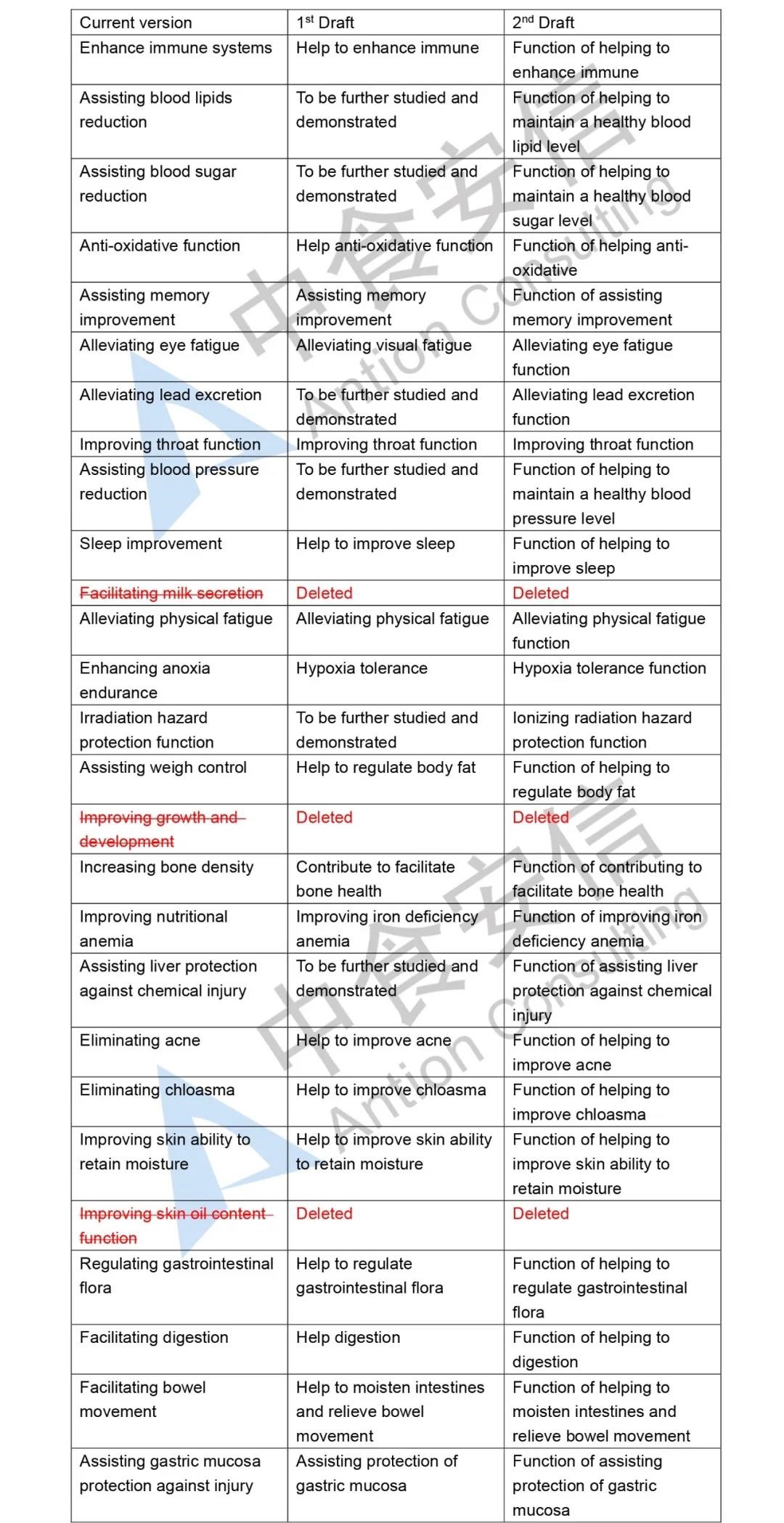
1. Adjust the Catalogue of Health Functions Allowed for Health Food Claims
SAMR once issued an announcement on soliciting opinions on adjusting the health functions of health foods on March 28, 2019. SAMR made adjustment based on the feedback, and this is the second time to solicit opinions. The purpose is to further strengthen the management of health food function claims and avoid false propaganda.
Majority adjustments include:
1.1 Eliminate three categories of health functions
The Draft adjusts the original 27 categories of health functions into 24 categories, and eliminates the original three categories of health functions, which includes “facilitating milk secretion”, “improving growth and development”, and “improving skin oil content”. Meanwhile, since the former Ministry of Health no longer accepts the approval of such health functions as suppressing tumor, assisted suppressing tumor, anti-mutation and anti-aging, these health function claims are not included in the Catalogue.
1.2 Standardize the expression of health functions
The Draft regulates the expression of the remaining 24 categories of health functions. For example, “assisting blood sugar reduction” is standardized as “function of helping to maintain a healthy blood sugar level”, and “assisting blood pressure reduction” is standardized as “function of helping to maintain a healthy blood pressure level”, etc. And the expression “function of helping to…” is added to most of the original health function claims. It not only solves the problem of false or exaggerated propaganda of health food in the market due to inaccurate expressions of health functions, but also guide consumers to correctly understand the function of health food to a great extent.
Figure 1 Comparison figure of Catalogue of Health Functions Allowed for Health Food Claims Non-Nutrient Supplements (2020 Edition) (Draft for Comments) (current version vs two editions of draft)
2. Increase the Interpretation of Health Function Claims
The Interpretation of Health Food Function Claims (2020 Edition) (Draft for Comments) increases the interpretation of claims for 24 categories of health functions. Previously, opinions were also solicited on the interpretation of health function claims for nutrient supplements health food. This can be said to be in line with the interpretation of health function claims for nutrient supplements. However, the Draft does not indicate that the interpretation can be claimed on the labeling.
3. Fill the Gap of Missing Evaluation on Some Health Food Functions
In addition to the adjustment of health functions, the Draft also releases the Evaluation Method of Health Food Functions (2020 Edition) (Draft for Comments) and Guidelines for Evaluation of Health Food Functions (2020 Edition) (Draft for Comments), which fill the gap that there has been no evaluation method for some health food functions.
4. Further Improve the Regulatory System of Registered Health Food
The issuance of Guidelines for Ethical Review of People Trial of Health Food (2020 Edition) (Draft for Comments) further improves the regulatory system of registered health food, which means that the work related to the ethics of people trial of health food has been clarified at the level of regulations, and also indicates that the regulatory system of registered health food is becoming more and more perfect.
Conclusion
The issuance of the Draft has a large impact on registered health food. It not only fills in the lack of regulations, but also gradually improves the legal system, which is conducive to the rapid development of the health food market. In addition, combined with the common problems and shortcomings in the current health food market, the Draft also makes relevant deletions and adjustments to health functions, which is more convenient for consumers to understand and also helpful for enterprises’ promotion, a win-win situation for consumers and enterprises, and more conducive to the healthy development of the whole industry. Through the interpretation of the Draft, Antion expects to help enterprises and industry personnel better grasp the changing trends and provide guidance for the next step in the work. Besides, Antion can provide consulting services such as consultation of standard and regulation, and food labeling review, etc. If you have any questions, please feel free to contact us!
More information about Catalogue of Health Functions Allowed for Health Food Claims Non-Nutrient Supplements (2020 Edition) (Draft for Comments), please click 【原創】医脉经纬(北京)管理咨询有限公司解讀《允許保健食品聲稱的保健功能目錄 非營養素補充劑(2020年版)(征求意見稿)》