2020 Industry Report for Infant Formula Milk Powder is coming out, which is published by Antion every year. The main contents of the report include the development status of infant formula milk powder market, summary of infant formula milk powder regulations standards and interpretation of important regulations, summary and analysis of infant formula milk powder quality status, and infant formula milk powder company dynamics. Here are some excerpts, to read the whole contents, welcome to contact us!

In 2020, China imported a total of 3.37 million tons of various types of dairy products, an increase of 10.2% year-on-year, with value of $12.50 billion, up 6.5% year-on-year. Among them, the import amount of whole milk powder in 1.31 million tons, down 3.3% year-on-year, the value is $8.36 billion, up 0.5% year-on-year; the import amount of infant and young children formula milk powder is 0.335 million tons, down 3.1% year-on-year, the value is $5.07 billion, down 2.4% year-on-year.
In 2020, in terms of infant and young children milk powder, the food regulatory agency of the State Council issued a total of 34 relevant regulations; 49 drafts for comments; 32 relevant food standards, of which 21 are national food safety standards, accounting for 55% of the total number of national food safety standards issued in 2020. Local food regulatory agencies issued a total of 39 relevant regulations.
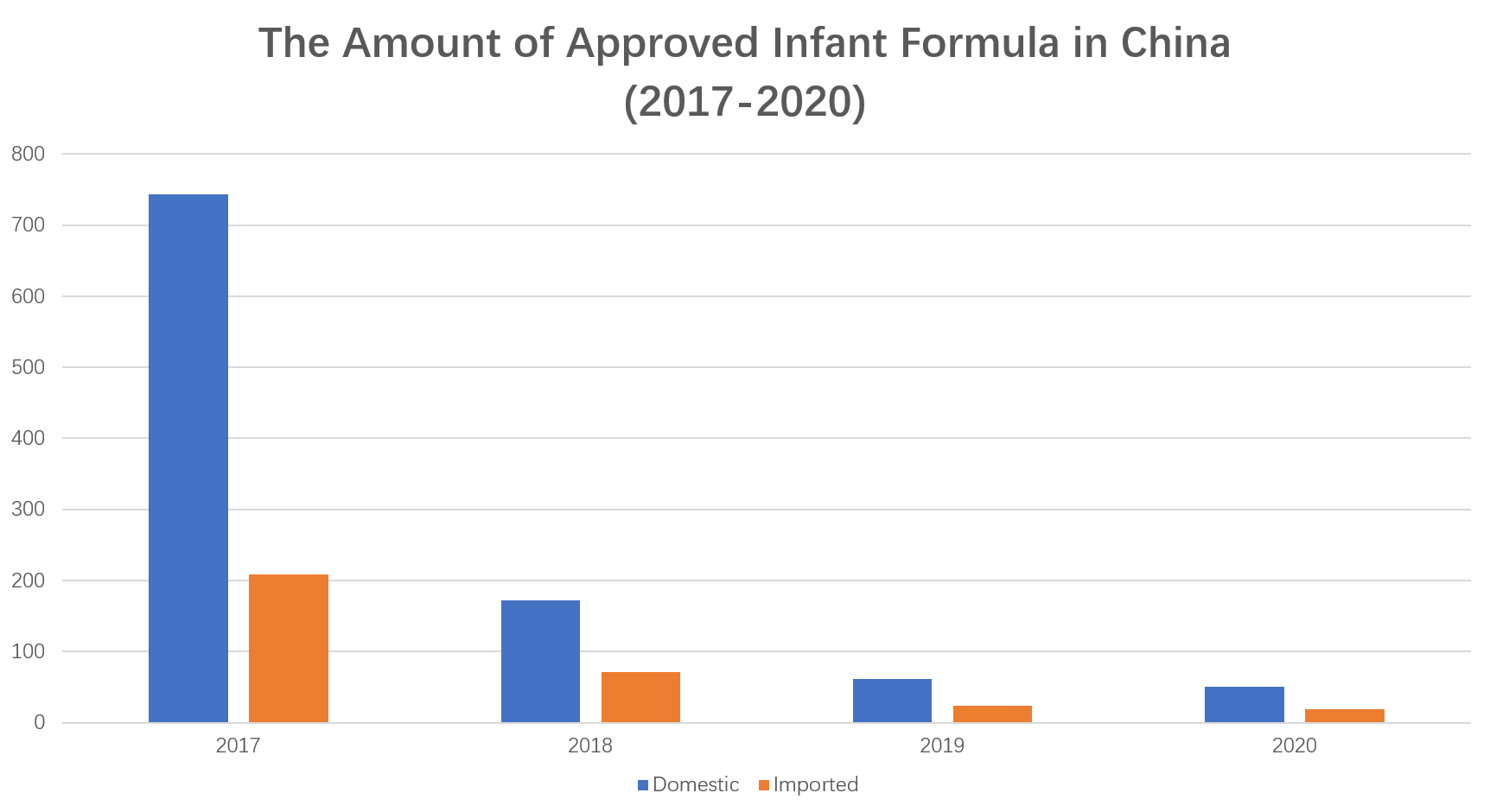
In 2020, SAMR totally approved 70 applications for registration of infant and young children formula milk powder products, including 11 domestic enterprises, with a total of formulas; 4 oversea enterprises, with a total of 19 formulas. The outbreak of COVID-19 has had a tremendous impact on the food industry, and the infant formula milk powder industry is no exception, especially the on-site review of formula registration. SAMR issued a notice in February to postpone the on-site review of special food registration during the epidemic prevention and control period, which undoubtedly delayed the progress of the registration and approval of infant and young children formula milk powder formula, especially for overseas manufacturers.
In 2020, NHC issued 10 new versions of food nutritional fortification substance standards including GB 1903.43-2020 National Food Safety Standard Food Nutrient Fortifier Cyanocobalamin, which is more in line with the actual situation of products, and caters to the development of the industry. It’s worth noting that all of these 10 food nutritional fortification substances can be used in infant formula milk powder, which is good news for enterprises, prompting the industry to be more standardized and facilitating the implementation of enterprises.
If you want to get the whole report, please contact us!
Lillian Fan
Tel: 010-51301566
email: Lillian.fan@ieasytrip.com
Relevant Reading
Changes in Chinese Infant and Follow-Up Formula Draft Standards
Introduction of Food Related Application & Registration Services