I. Mandatory Labeling Contents
The labeling of infant and young children formula milk powder should indicate the product information, enterprise information, usage information, storage conditions, etc. used to identify the characteristics and safety warnings of infant formula and young children milk powder.
(1) Product information
Infant and young children formula milk powder product information includes food name, ingredients list, nutrition information, specification (net contents), registration number, product standard code, production date, and shelf life.
The product name consists of commodity name and common name. Each product can only have one product name. The product name should use standard Chinese characters. "Standardized Chinese characters" refers to the Chinese characters in the Common Standard Chinese Characters Table, excluding traditional Chinese characters, letters, figures, symbols, etc. The imported infant formula milk powder can also be marked with English name, which should be corresponding to Chinese name.
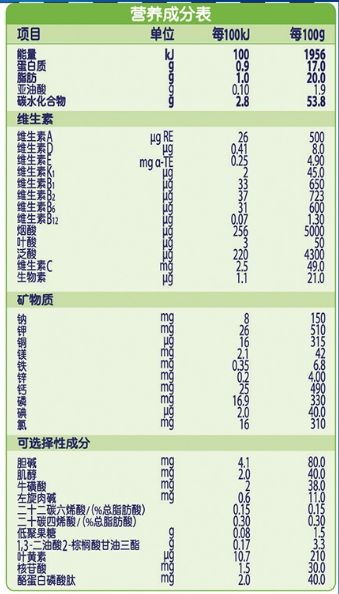
The nutrition information should be consistent with the content and order of the application for registration, and should be classified and listed according to energy, protein, fat, carbohydrate, vitamin, mineral, optional ingredient and other categories. The nutrition information should be marked according to the content in every 100 kJ and 100 g, and the content in every 100 mL can be marked at the same time. The nutritional components should be listed in the order specified in National Food Safety Standard Infant Formula (GB 10765) and National Food Safety Standard Older Infants and Young Children Formula (GB 10767). In addition to the provisions of GB 10765 and GB 10767, they should be listed in the order specified in the National Food Safety Standard for the Use of Nutritional Fortification Substances in Foods (GB 14880).
(2) Enterprise information
The enterprise information of infant formula milk powder includes the manufacturer name, production address, production license number (if any), contact information, and the enterprise information marked should meet the following requirements:
The labeling of imported infant formula milk powder should also indicate the country (region) of origin, as well as the name, address and contact information of agents, importers or distributors register according to law in China. Where an overseas production enterprise of imported food has obtained registration, the registration number should be indicated.
(3) Usage information
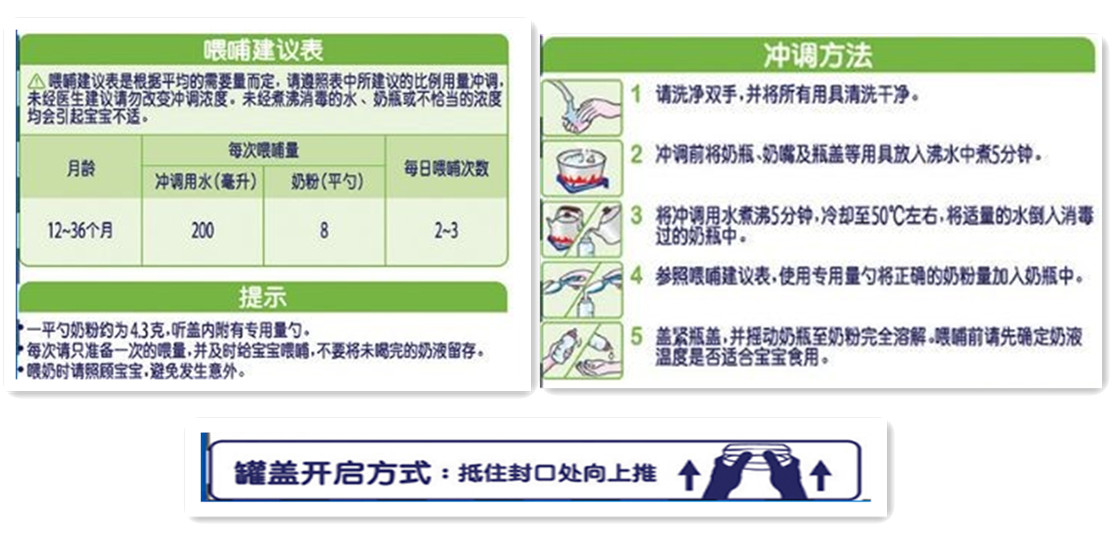
The use of infant and young children formula milk powder should include the method of consumption and dosage:
Indicate the method of consumption, daily or per meal consumption, preparation instructions and diagrams, and if necessary, the opening method of the container should be labeled. When the maximum surface area of the package is less than 100 cm2 or the product quality is less than 100 g, the diagram is not necessary.
The instruction (tips) should give warning to the possible health hazards caused by improper preparation and usage.
(4) Storage condition
If necessary, the storage conditions after opening should be indicated. If the unsealed product is not easy to store or is not suitable to store in the original packaging container, special tips should be given to the consumer.
(5) Other requirements
The labeling of infant formula milk powder should indicates: "The most ideal food for infants is breast milk for infants of 0-6 months; when breast milk is absent or note enough, this product can be used"; the labeling of older infant formula food should indicates "auxiliary food must be added".

If the source of raw milk or raw milk powder is indicated, the specific place or country of origin should be indicated truthfully.
The labeling of infant formula milk powder should also indicate other items or information that need to be indicated according to laws, regulations or national food safety standards.
II. Optionally annotated content
The optional contents of the labeling include: content claim and function claim allowed by the national food safety standard, as well as information used for product traceability, reminder or warning, product after-sales service, etc. Among them, the national food safety standard of our country clearly stipulates the content value of the essential components (such as protein, fat, carbohydrate, vitamin B1, vitamin B2, vitamin C, calcium, iron, zinc, etc.) in the 0-6-month-old infant formula milk powder, so the infant formula milk powder must meet the content requirements of the standard, and should not claim the content and function of the essential components.
III. Prohibited content
(1) The contents concerning function of disease prevention and disease treatment
(2) The contents explicitly or implicitly declaring the function of health care;
(3) The contents explicitly or implicitly declaring the functions such as “be beneficial to intellectual development”, “increase the resistance or immunity” and “protect the intestinal function”, etc;
(4) The words such as “no adding”, “no containing” and “additive free”, etc. used to emphasize no use or no containing of a certain substance that should not be used or contained in the product formula according to the food safety standard;
(5) False, exaggerated and absolute contents and those in violation of scientific principle;
(6) The fuzzy information such as "imported milk source", "from foreign pastures", "ecological pastures", "imported raw materials", "original ecological milk source" and "pollution-free milk source" is used as raw material source;
(7) Claims inconsistent with the content of product formula registration
(8) Using images of babies and women, "human emulsification", "breast-feeding" or similar terminology;
(9) Other circumstances that do not conform to the regulations.
IV. Reference regulations
l GB 7718-2011 National food safety standard General Standard for the labeling of prepackaged foods
l GB 28050-2011 General Rules for the Nutrition Labeling of Prepackaged Foods
l GB 10765-2010 National Food Safety Standard Infant formula
l GB 10767-2010 National Food Safety Standard Older Infants and Young Children Formula
l Technical Guidelines for Formula Registration Labeling of Infant Formula Milk Powder (Trial)