In March, 2021, the NHC issued National Food Safety Standard Infant Formula (GB 10765-2021), National Food Safety Standard Older Infants Formula (GB 10766-2021) and National Food Safety Standard Young Children Formula (GB 10767-2021) (hereinafter referred to as the New Standards). The infant formula industry has also ushered in a new stage of quality upgrade.
The New Standards have made clearer provisions on protein, carbohydrates, trace elements and optional ingredients, and have left a 2-year transition period for infant formula manufacturers. During this period, infant formula enterprises need to produce in accordance with the New Standards as soon as possible, and related regulatory authorities will conduct inspections and reviews on the products produced in accordance with the New Standards.
Since the issuance of the New Standards, some leading enterprises of infant formula milk powder are starting to select raw and auxiliary materials for new products, design new formulas and innovative research and development, and adjust production process and technologies under the guidance of the New Standards.
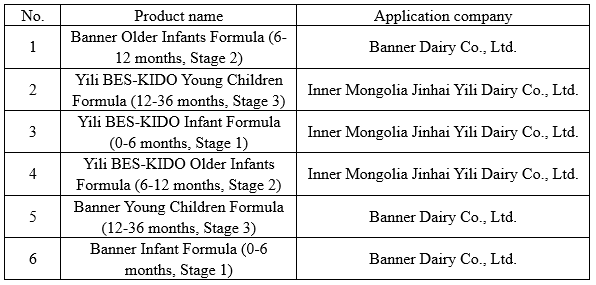
On March 2, 2022, SAMR released the information on the Formula Registration Result of Infant and Young Children Formulas Milk Powder, including Yili BES-KIDO infant and young children formula and Junlebao Banner infant and young children formula.
From the overall revision, New Standards put forward more stringent requirements for product quality requirements. Nutrient indicators have been adjusted. New Standards sets maximum values for all nutrients of older infants formula. And refine product standards and split the current 2 standards into 3 standards to enhance the scientific nature.
The New Standards modified terms and definitions, pointing out that protein sources of dairy-based and soy-based product must not be mixed; adjusted protein and carbohydrate indicators, in which new indicators of carbohydrate-related requirements for older infants formula and young children formula are added, and older infants formula shall not use fructose and sucrose as a source of carbohydrates; and improved the vitamin and mineral indicators, for infants formula, the vitamin and mineral indicators are adjusted.
More information about the changes of the New Standards, please click New Standards of Infants Formula Landing.
If you are interested in the registration of infant and young children formula, please feel free to contact us!
Hongtao Fei
Tel: 010-51301566
email: feiht@ieasytrip.com
Source: Antion & SAMR
Note: This article is compiled by Antion, please indicate our source if reprint it.