On October 8, 2021, the Center for Food Evaluation of State Administration for Market Regulation issued regulations on health food. The labeling requirements for precautions and unsuitable people for health food using different ingredients, selection and confirmation of test methods for iconic components of probiotics and the requirements for document literature during the application and approval were stipulated. Antion summarized some contents as follows:
The labeling requirements for precautions for health food using different raw materials
Due to the limitations of raw materials, the precautions for health food using different ingredients should and are not limited to the following labeling contents:
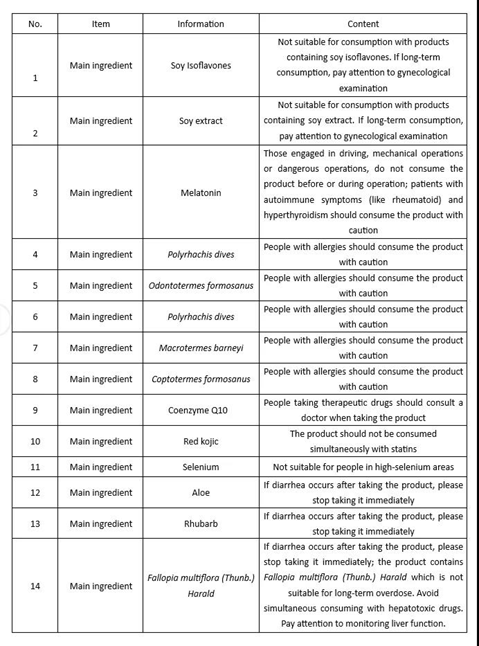
The labeling requirements for unsuitable people for health food using different ingredients
Due to the limitations of raw materials, the unsuitable people for health food using different ingredients should and are not limited to the following labeling contents:
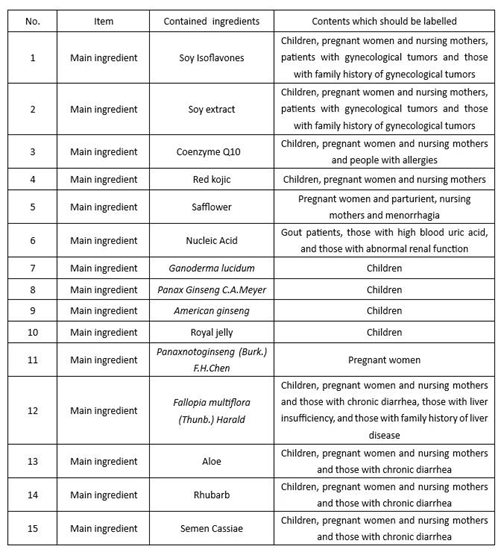
Selection and confirmation of test methods for iconic components of probiotics in health food
In view that the current national and international standards for test methods for probiotics can basically meet the needs of probiotics testing of health food, and the test methods in national and international standards are stable, reproducible, and applicable, the research documents of test methodology for iconic components of probiotics are not currently being seen as a mandatory requirement, but the test methods selected and confirmed by the registration applicant should comply with the following national or international standards:
(1) For the testing of probiotic products whose tested strains are in the current List of Probiotic Strains Available for Health Food and List of Strains Available for Food, the test methods must adopt the methods specified in GB 4789.34 or GB 4789.35.
(2) For products whose tested strains are not in the current List of Probiotic Strains Available for Health Food and List of Strains Available for Food, and the testing cannot adopt the methods specified in GB 4789.34 or GB 4789.35, AOAC (Association Of Official Analytical Chemists) microbial inspection method system, USDA (United States Department of Agriculture) microbial inspection method system, IDF (International Dairy Federation) microbial inspection method system, FDA Bacteriological Analytical Manual (BAM) and methods specified in ISO 16140 can be adopted.
(3) For products whose tested strains are not in the current List of Probiotic Strains Available for Health Food and List of Strains Available for Food, and which adopt self-developed test methods, the basis for selection and determination of test methods should be provided, and comparing with the test results of three batches of products according to the corresponding methods of the above-mentioned international microbial inspection method system.
The requirements for document literature during the application and approval of health food
The document literature includes scientific research papers officially published in domestic core professional journals or international professional journals; related descriptions of traditional Chinese herbal classics; literature analysis and evaluation reports; international standards, national standards, risk assessments and statistical information officially released by internationally recognized food hygiene authorities or organizations, or China’s authorities or related departments.
In addition, there are also related regulations on FAQs and precautions of the technical requirements for health food, the guidelines of stability test, and the selection and verification requirements for sterilization process conditions, so that health food enterprises can better carry out their work in accordance with these regulatory requirements.
If you are interested, please feel free to contact us!
Source: CFE of SAMR
Note: This article is compiled by Antion, please indicate our source if reprint it.